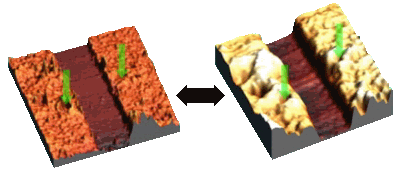
Above: Scanning force microscopy images of a polymethacrylic acid brush in water at two different values of pH are shown above [1].
Polyelectrolytes have the interesting property of changing their conformation in solution depending upon whether the solution is acid or base. Normally in water or base a polyacid will donate a proton to create a hydronium ion (H3O+); however in an acidic environment, these ions are in abundance, so the polyacid keeps its proton, which usually renders it hydrophobic. A hydrophobic polymer in aqueous solution will coil up to exclude the water. In base, the de-protonated polyacid is positively charged and will swell due to the Coulombic repulsion of the negative charges. If the acid is chemically grafted to a surface (a brush; see the figure above right) the swelling is caused by the osmotic pressure of the counterions trapped within the brush. Polyelectrolyte brushes have a unique property that might be used in various nanotechnologies: they are responsive. Swollen and collapsed brushes have very different properties: Swollen brushes can be used to help suspend inorganic nanoparticles in solutions; collapsed brushes may cause aggregation of the particles. Collapsed brushes may have particles encased within them; swollen brushes may release these particles. The tribology (wear or frictional properties) of such surfaces are very different. We are using a variety of experiments to characterise such polymers and to develop ideas for technologies where they might be used [1]. We have studied the structure of polyelectrolyte brushes [2], and have shown how they can be used for switchable adhesion [3].
More about switchable adhesion
References
[1] A. J. Ryan et al. Faraday Disc. 128 55-74 (2005).
[2] M. Geoghegan et al. Soft Matter 2 1076-80 (2006).
[3] R. La Spina et al. Angew. Chem. Int. Ed. 46 6460-3 (2007).