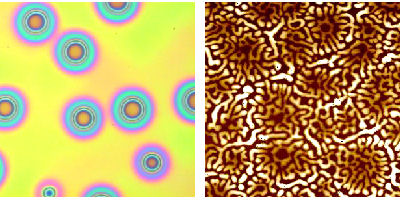
Above: The optical microscopy image of polystyrene dewetting PMMA on the left is 150 µm in length. The image on the right is an SFM image 10 µm in length. The bright regions are some 50 nm higher than the dark regions. Here a 30 nm PMMA layer (subsequently removed) has dewetted a 15 nm polystyrene film. The image shows the remaining polystyrene structure.
Polystyrene and poly(methyl methacrylate) (PMMA) are ideal polymers for the study of dewetting, because they are easy to work with, and have similar glass transition temperatures. One way a film might break up is by nucleation and growth of holes, initiated by long-range (van der Waals) forces. This occurs when the surface energy of the lower film is less than the sum of the interfacial energy and the surface energy of upper film. An example of the former is shown in the optical microscopy image above left. In this case, polystyrene dewets PMMA with the formation of holes randomly distributed throughout the film. The holes will all exhibit the same growth, which is dependent upon the thickness of the film, and the viscosity of the polymers [1].
Van der Waals forces are long-range forces which act between all molecules. They are also always attractive. If the van der Waals interaction between an upper layer and a substrate is more attractive than that between the substrate and the lower layer, a bilayer film can break up. However, there are many parameters that can alter this; film thickness is a major one, because the strength of the interaction between two films decreases with their distance apart. If the layers are all thin (several nm) then minor inhomogeneities in the surfaces may create unusual structures, possibly like those in the scanning force microscope (SFM) image above. In this case, a PMMA layer on polystyrene is unstable. The films are annealed, and the PMMA is removed using acetic acid. In this case, it is difficult to ascertain which film is unstable. The stability of such bilayers as the thickness is changed has recently been investigated by Johann de Silva [2].
References
[1] C. Wang, G. Krausch, and M. Geoghegan, Langmuir 17 6269-6274 (2001).
[2] J. P. de Silva, M. Geoghegan, A. M. Higgins, G. Krausch, M.-O. David, and G. Reiter Phys. Rev. Lett. 98 267802 (2007).