Leishmaniasis is responsible for the deaths of up to 40000 people each year. It is a disease caused by the parasite Leishmania, which is transmitted by the bite of a female sand fly. The parasite lives in the sand fly for about a week during which time it goes through different lifecycle development stages. The sand fly, which feeds on blood, picks up the parasites when it bites an infected vertebrate (typically, dogs, rats, or humans). At that point, the parasites are amastigotes, which means that they don't have a flagellum, which is the "tail" that you can see in the animation, and you may make out one going diagonally down from the top left in the image directly below. The parasites must adhere to the sand fly midgut in order to survive and develop. Most don't adhere, which is why interfering with this adhesive process is of such interest. If most don't adhere, then perhaps this is a good point to interfere with their life cycle. Successful parasites grow flagella, at which point they become promastigotes. These "nectomonads" stick to the sand fly gut wall until such point as they undergo metacyclogenesis, which means that they become infectious. These parasites are then able to leave the sand fly the next time it bites another human or an animal. And then the parasite enters into the next stage of its lifecycle.
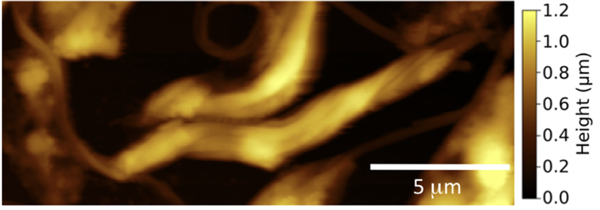
Above: This is a scanning force microscope (usually called AFM) image of Leishmania mexicana parasites in air. The parasites are not viable in air, but images obtained this way show their elongated shape quite well. The colour scale represents height differences in the image. Lighter parts are elevated with respect to darker parts. All lengths are denoted in microns, where a thousand microns is a millimetre. The image is taken from our paper, cited below.
The question remains as to how the parasite sticks to the sand fly midgut. The parasite is a protozoa, which means it is a single cell with a nucleus. The outer coating of the cell is its glycocalyx, the main component of which is lipophosphoglycan (LPG). It is this sugar-coating which is known to be responsible for parasite adhesion in the sand fly. We have shown that this LPG adheres very strongly to a galectin that is similar to one present in the sand fly midgut. Not only that, but we showed that when the parasite has undergone metacyclogenesis, its adhesion is weaker. This is of course important, if the parasite is to leave the sand fly to start the next part of its lifecycle.
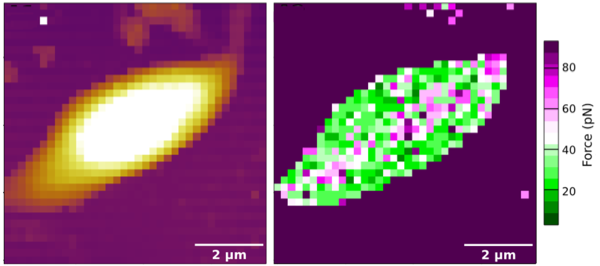
Above: The figure above left shows a topography (height) image of a Leishmania mexicana parasite using a force spectroscopy experiment. The resolution of this experiment is poor because it is in air, and because force spectroscopy requires more time to get a nice image than scanning force microscopy. The right hand image presents the same experiment, but this time showing the adhesion at each pixel rather than the height. The colour of each pixel represents the adhesion of the AFM tip to the parasite. In these two images the tip was coated with sugars. The chemistry to do this is remarkable. The tips have a nominal radius of about 20 nm; the 2 micron scale bar is 100 times bigger than this! The regions of high adhesion to the sugar-coated tip in this parasite seem to be restricted to a central line, which we think might be significant. These images were taken from Amy's thesis, with her permission, although the one on the right is also in our paper, cited below.
We measured Leishmania adhesion by using an atomic force microscope. These contain nanoscale tips that can measure adhesion of a few pN. (The force that an average UK woman exerts standing on the floor is about 700 N. This is about ten trillion times the force that an AFM can easily measure.) The AFM contains a tiny nanoscale tip that is attached to its controller via a cantilever support. When the tip makes contact with a surface, the cantilever bends a little. This bending can be measured, and from this the force of interaction calculated. It's all very clever and was worth a Nobel prize for its inventors. Amy was able to synthesize glycopolymers (sugars) on AFM tips and the force in retracting them from the parasite was measured. If this sounds difficult, well, frankly it is. And Amy did a splendid job of it.
This project was done in collaboration with Dr Matthew Rogers at the London School of Hygiene and Tropical Medicine and Professor Neil Cameron, who is now at Monash University. I met Matt some time ago, and I explained a little about what AFM could do. He immediately grasped the potential of the technique and asked if I could measure adhesion with sugars, which I couldn't because glycosylation of polymers is not trivial. But I was intrigued by the biological question that Matt was asking and I knew that Neil had the expertise that we needed. Fortunately, Neil was up for it and suggested that his (then) postdoctoral researcher Dr Ahmed Eissa help Amy. Also fortunately, Neil was then at Durham University, for which travel was a lot easier from Sheffield than Monash. So Amy took this chemistry back to Sheffield. She learnt to look after her cryo-frozen parasites with help from colleagues in the Medical School and her cosupervisor (Professor David Dockrell). The idea to work with Leishmania was given to me by a rather eminent scientist and physician, Professor Michel Bergeron, at a meeting at the Indian Institute of Technology in Kanpur. The idea appealed to me, because very little was being done in this or related areas in the biophysical sciences and it just seemed so exotic!
A. R. Hall, J. T. Blakeman, A. M. Eissa, P. Chapman, A. L. Morales-García, L. Stennett, O. Martin, E. Giraud, D. H. Dockrell, N. R. Cameron, M. Wiese, L. Yakob, M. E. Rogers, and M. Geoghegan "Glycan-glycan interactions determine Leishmania attachment to the midgut of permissive sand fly vectors" Chem. Sci. 11 10973-83 (2020)
The article is free-to-read.